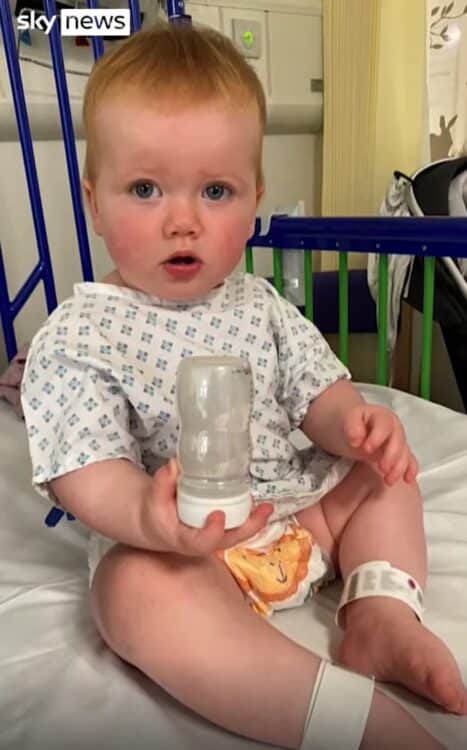
In a remarkable breakthrough, a British toddler, Opal Sandy, has experienced a life-changing transformation, becoming the first individual worldwide to undergo a revolutionary gene therapy trial that restored her hearing. Opal, who was born unable to hear due to auditory neuropathy, a condition stemming from a faulty gene disrupting nerve impulses from the inner ear to the brain, now enjoys near-perfect hearing, thanks to this pioneering treatment.
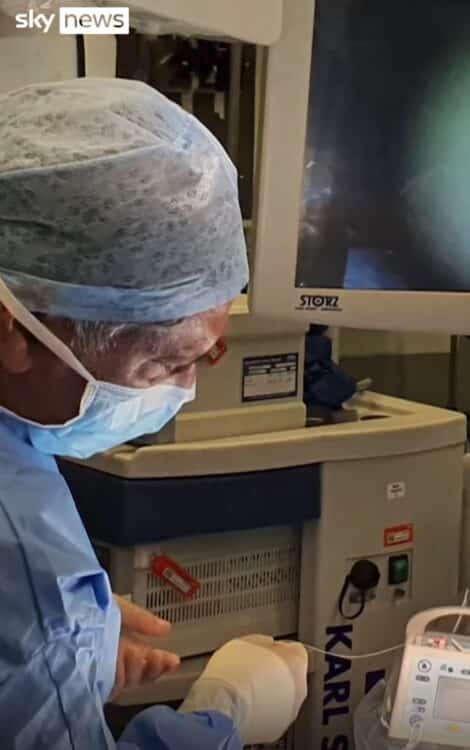
The groundbreaking surgery, completed in a mere 16 minutes, involved administering an infusion containing a functional copy of the gene to Opal. The outcome left her parents astounded when they witnessed her responding to sounds for the first time post-treatment. Jo Sandy, Opal’s mother, described the moment as surreal, emphasizing the sheer disbelief at witnessing her daughter’s newfound ability to hear.
Conducted at Addenbrooke’s Hospital, part of Cambridge University Hospitals NHS Foundation Trust, the trial, named Chord, holds immense promise for transforming the lives of deaf individuals globally. Led by Professor Manohar Bance, an ear surgeon at the trust, the trial aims to evaluate the efficacy of gene therapy in addressing auditory neuropathy caused by OTOF gene mutations.
Opal’s success story marks a significant milestone, with her response to the therapy demonstrating outcomes surpassing expectations. Professor Bance expressed optimism about the trial’s potential to offer a cure for patients grappling with similar forms of deafness, underscoring the extensive research and dedication culminating in this groundbreaking achievement.
The gene therapy, developed by biotech firm Regeneron, entails delivering a functional gene copy via a harmless virus to rectify the underlying genetic fault. Opal’s experience heralds a new era in deafness treatment, offering hope to countless families navigating similar challenges.
The Chord trial, comprising three phases, aims to assess the safety and efficacy of gene therapy across a cohort of 18 children globally. Opal’s pivotal role as the first recipient underscores the trial’s significance in paving the way for future advancements in treating auditory neuropathy.
Martin McLean of the National Deaf Children’s Society emphasized the importance of such breakthroughs in ensuring deaf individuals lead fulfilling lives. He welcomed the developments, emphasizing the need for continued research to ascertain the long-term outcomes for children undergoing such treatments.
While Opal’s hearing has been restored, her parents now face the delightful challenge of managing their daughter’s newfound love for creating noisy antics, a testament to the joyous journey of her restored hearing.
Related Articles: