In Canada there is an eight-step vaccine safety program that is in place to test the vaccine’s potency, safety and purity before release.
Vaccines are one of the most monitored and studied tools used in medicine today because they are given to healthy people and mainly to children.
Before any vaccine is authorized for use in Canada, it undergoes extensive testing to ensure that it meets the high standards set by BGTD for quality, safe and effective. And testing does not stop once a vaccine is authorized for use. Once a vaccine is authorized for use, studies and monitoring continue to alert health professionals to any potential issues with a particular vaccine.
But even with these very stringent rules and testing some children and adults have adverse effects to the doses.
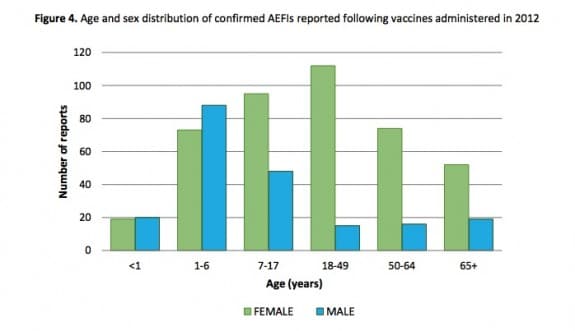
During their first report on Vaccine Safety in Ontario, Public Health Ontario looked at vaccines administered between January 1 and December 31, 2012 to track adverse events following immunization(AEFI).
The agency’s director of immunization and vaccine preventable diseases, Dr. Shelley Deeks, told CBC News the goal is to be transparent about vaccines in a climate where there are many questions and misperceptions about the products.
“I feel we have a responsibility to provide this information back [to the public] … and probably do a little bit of a better job of communicating how safe our vaccines are,” Deeks says.
“But also communicating that they’re not perfect, and that no drug or biologic product is perfect.”
Their report states that there were 631 reports of confirmed AEFIs representing an overall reporting rate of 4.7 per 100, 000 population. The number of reports increased in 2012 compared to the previous two years; however, the rate of reporting in 2012 relative to population size in Ontario is lower than some other jurisdictions.
Of the 7.8 million vaccinations that were administered, there were 56 serious vaccine-related adverse events reported in 2012, which equals 7.2 serious events for every one million vaccinations.
Serious reactions were things like convulsions/seizures and anaphylaxis, an allergic reaction that can range from an itchy rash and hives to difficulty breathing and in severe cases shock.
Children, and teens 18 and younger accounted for just over half of adverse events.
The most frequently reported agents among all confirmed AEFI reports were
- Inf – influenza or flu shot (28.7%),
- Tdap – tetanus, diphtheria, acellular pertussis(9.7%),
- DTaP-IPV-Hib – diphtheria, tetanus, acellular pertussis, inactivated polio,Haemophilus influenza e type b(9.0%)
- DTaP-IPV – diphtheria, tetanus, acellular pertussis, inactivated polio(8.4%)
- Var – chickenpox (8.4%)
- HB -hepatitis B(7.9%)
- HPV4 -human papillomavirus quadrivalent(7.3%)
- Pneu-C-13 – pneumococcal conjugate 13-valent(6.7%)
- Pneu-P-23 – pneumococcal polysaccharide 23-valent(6.3%)
- MMR – measles, mumps, rubella (5.2%)
Among the 56 serious AEFI reports, 57.1% (n=32) were related solely to a medically important event, 30.4% were related solely to a hospital admission of >24 hours (n=17) and 12.5% (n=7) had both a hospital admission of >24 hours and a medically important event. Among all hospital admissions (n=24), the median length of stay was 2.5 days (range 1 to 18 days).
The vaccine for which almost a third of the adverse events reported was influenza. But Ms. Deeks says that can be explained by the sheer volume of the province’s flu shot program.
Some of the issues that were noted were vaccinations being administered to children outside of the recommended age group, likely because the children were late getting their shots and needed to catch up. This was seen for DTaP- IPV(Quadracel®) vaccine, which accounted for more than 8.4% of all AEFIs.
It was also found that in children, between the ages of 1-6, the Varivax vaccine(chickenpox) was sometimes administered in the wrong site(thigh). If your child is over a year old, this vaccine should be injected in the outer aspect of the upper arm.