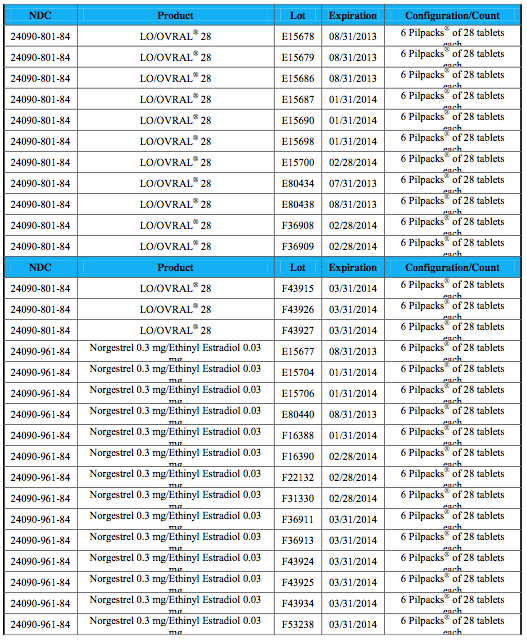
As a result of this packaging error, the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy. These packaging defects do not pose any immediate health risks. However, consumers exposed to affected packaging should begin using a non-hormonal form of contraception immediately. Patients who have the affected product (lot number are provided below) should notify their physician and return the product to the pharmacy.
Any adverse events that may be related to the use of these products should be reported to Akrimax Medical Information at 1- 877-509-3935 (8 AM to 7 PM Mon-Fri CST) or to FDA’s Med Watch Program either online, by regular mail or by fax.
Related Articles:
- Johnson & Johnson Recalls Aveeno Baby Calming Comfort Lotion
- Britax Announces Voluntary Safety Recall of the Chaperone Infant Car Seat
- Bumbleride Offers Wheel Retrofit Kit For Indie & Indie Twin
- President’s Choice Recalls Organic Baby Powdered Cereal; May Be Rancid
SOURCE: Pfizer Press Release