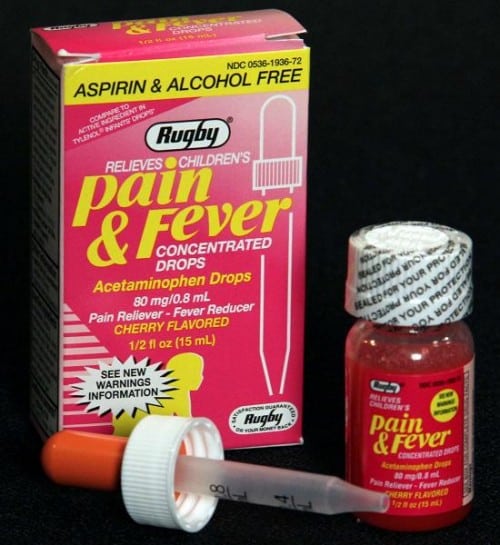
The U.S. Consumer Product Safety Commission, in cooperation with Altaire Pharmaceuticals, today announced a voluntary recall of about 898,000 bottles of Children’s Pain & Fever Concentrated Drops because this over-the-counter medicine contains acetaminophen which calls for child-resistant packaging as required by the Poison Prevention Packaging Act. Although the original bottle has child-resistant packaging, a separate dropper unit provided for dispensing the drug to children does not. When in use, a child can access the medicine, posing serious health problems or death if more than the recommended dosage is consumed.
The recall involves Rugby Children’s Pain & Fever Concentrated Drops (Acetaminophen Drops) in a 1/2 fl. oz. (15 ml) bottle size. The UPC code 305361936723 can be found with the bar code at the bottom of the box.
The lot numbers can be found stamped into the bottom of the carton with the expiration date and above the label on the bottle printed in black.
| 09002 | 09379 | 10272 | 10368 | 10487 |
| 09131 | 09394 | 10273 | 10406 | 11058 |
| 09215 | 10154 | 10366 | 10433 |
Consumers should immediately store this product with the child-resistant closure in place and keep it out of the reach of children. To arrange for a free replacement dropper, contact Altaire Pharmaceuticals at (800) 258-2471 9 a.m. to 5 p.m. ET Monday through Friday.