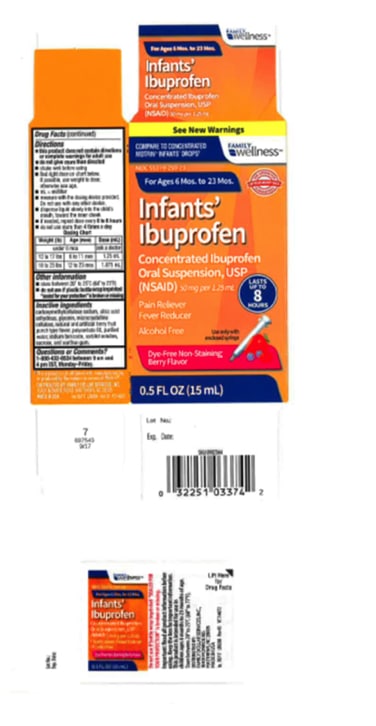
Parents! Check your cupboards. Tris Pharma, Inc. has voluntarily recalled three (3) lots of Infants’ Ibuprofen Concentrated Oral Suspension, USP (NSAID) 50 mg per 1.25 mL, to the retail level. The recalled lots of the product have been found to potentially have higher concentrations of ibuprofen.
There is a remote possibility that infants, who may be more susceptible to a higher potency level of drug, and therefore may be more vulnerable to permanent NSAID-associated renal injury. Adverse effects that may be experienced are nausea, vomiting, epigastric pain, or more rarely, diarrhea. Tinnitus, headache and gastrointestinal bleeding are also possible adverse effects. To date, Tris Pharma, Inc. has not received any reports of adverse events related to the lots of product that are the subject of this recall.
The product is used as a pain reliever/fever reducer and was packaged in 0.5 oz. bottles for the recalled lots listed below:
| NDC | LOT | EXPIRATION | DESCRIPTION | COMPANY |
| 49035-125-23 | 00717009A
00717015A 00717024A |
02/19
04/19 08/19 |
Equate: Infants’ Ibuprofen Concentrated Oral Suspension,
USP (NSAID), 50 mg per 1.25 mL, 0.5 oz. bottle |
Wal-Mart Stores Inc |
| 59779-925-23 | 00717024A | 08/19 | CVS Health: Infants’ Ibuprofen Concentrated Oral Suspension,
USP (NSAID), 50 mg per 1.25 mL, 0.5 oz. bottle |
CVS Pharmacy |
| 55319-250-23 | 00717024A | 08/19 | Family Wellness: Infants’ Ibuprofen Concentrated Oral Suspension,
USP (NSAID), 50 mg per 1.25 mL, 0.5 oz. bottle |
Family Dollar Services Inc. |
Tris Pharma, Inc. sold the affected lots of Ibuprofen Concentrated Oral Suspension, USP (NSAID) 50 mg per 1.25 mL to one customer, which distributed the lots into the US market. Tris Pharma, Inc. has notified its customer by urgent recall notice and is arranging for the return of the recalled product.
Wholesalers and retailers of the product should stop further distribution of the affected lots of Ibuprofen Concentrated Oral Suspension, USP (NSAID) 50 mg per 1.25 mL, which are being recalled.
Consumers with questions regarding this recall can contact Tris Customer Service at 732-940-0358 (Monday through Friday, 8:00am ET- 5:00pm PT) or via email at Customer Service Email . Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of the product lots subject to this recall may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.ht…
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms…. or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.